potassium electron configuration full|Potassium (K) : Clark Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s . Compensation is a key element of a total rewards strategy. Recognized as the world’s standard since 1976, the Certified Compensation Professional (CCP) designation is known throughout the total rewards community as a mark of expertise and excellence in the fundamentals of compensation. The CCP designation demonstrates you possess .
potassium electron configuration full,Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium has an electron configuration of [Ar]4s 2. This gives .
Human ether-a-go-go related gene (HERG) potassium channels have uniquely rapid inactivation kinetics which are critical to the role they play in regulating cardiac electrical activity.In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom (there are 19 electrons). When we write the configuration we'll put all .
Mar 23, 2023 Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s .Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium has an electron .
Potassium (K) The basic elemental potassium is a silvery-white soft alkali metal that oxidizes instantly in the air and reacts vigorously with water. With water, it generates sufficient heat that ignites hydrogen which gets emitted in the .
potassium electron configuration full Potassium (K) The basic elemental potassium is a silvery-white soft alkali metal that oxidizes instantly in the air and reacts vigorously with water. With water, it generates sufficient heat that ignites hydrogen which gets emitted in the .In the Periodic table, potassium is one of seven elements in column (group) 1 (alkali metals):they all have a single valence electron in their outer electron shell, which they readily give up to .Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium has an electron configuration . Potassium Electron Configuration: Potassium is a chemical element. Its symbol is K that is taken from Neo-Latin kalium. The atomic number of potassium is 19. Firstly it was isolated from potash and the ashes of plants, .The full electron configuration of potassium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1; Shorthand Electron Configurations. Using potassium as an example again: The nearest preceding noble gas to potassium is argon; This accounts for 18 electrons of the 19 electrons that potassium has; The shorthand electron configuration of potassium is [Ar] 4s 1The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron . Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable. Next, determine .

Potassium is used in glass making and is found in fertilizers and soaps. Potassium is in group one, and is the 4th element down in it's column. This tells us that it is an alkali metal. It is very reactive, has a low density, and is a good reducing agent. Potassium can form an alloy with \(Na\) that has a low vapor pressure and melting point.

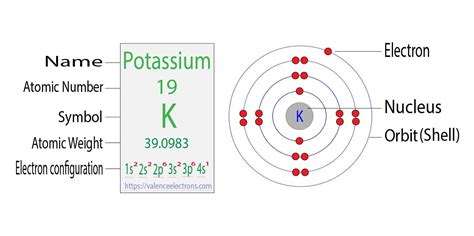
Draw the electronic configuration for potassium using the electron configuration diagram below. Remember that potassium is element number 19 so has 19 electrons. Every line in the energy diagram below holds 2 electrons of opposite spins. . partially due to the fact that the outermost energy level electron configuration was full (ns 2 np 6 . Potassium is the FIRST element in the FOURTH row of the table.So it's the same as Argon, but with one extra electron in its 4s subshell.Check me out: http://.That is why the full electronic configuration for potassium ends with 4s1, which means its valence electron resides in the 4s subshell. And the remaining 18 electrons are the core electrons that fill up each of the subshells that precede the 4s subshell or are lower in energy than the 4s subshell.
Electron configuration for potassium. Electron configuration. Shorthand configuration [Ar] 4s 1: Electron configuration. Full configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1: Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 4s 1: Electrons per shell: 2, 8, 8, 1: Valence electrons : 1: Valency electrons : 1: Bohr model:
Full Electron Configuration: Nobel Gas Shorthand: Neon: Z = 10: Ne: 1s 2 2s 2 2p 6: Ne: [He] 2s 2 2p 6: . the electron configuration of potassium, which begins the fourth period, is [Ar] 4s 1, and the configuration of calcium is [Ar] . The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the .
The arrangement of electrons in various energy levels of an element is called electronic configuration. The maximum number of electrons which can be accommodated in any shell of an atom is given by 2n 2. Atomic number of potassium = 19; Electronic configuration = 2,8,8,1 (K- shell = 2 electrons, L-shell = 8 electrons, M - shell = 8 electron, N .
Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable. Next, determine .
potassium electron configuration full Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . We have chosen to show the full, unabbreviated configurations to provide .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and 2p subshells .
Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable. Next, determine . What is the electron configuration of potassium? Chemistry Electron Configuration Electron Configuration. 2 Answers MAT Nov 16, 2015 #1s^2,2s^2,2p^6,3s^2,3p^6,4s^1# Answer link. Kazi Ashiq Iqbal Nov 16, 2015 2,8,8,1. Explanation: If you want to represent the subshells you can also write it in this way: .
Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. We’re hiring! Embed. Share via. Electron Configuration Calculator. . Read the full electron configuration: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 1 \rm 1s^2 2s^2 2p^6 3s^2 3p^6 3d^{10} .
Consequently, the electron configuration of potassium, which begins the fourth period, is [Ar]4s 1, and the configuration of calcium is . By definition, orbitals are most stable when they are either full or half-full. Thus, in some cases where valency is very near a stable configuration, the actual electron configuration of the element .
potassium electron configuration full|Potassium (K)
PH0 · Potassium dependent structural changes in the selectivity
PH1 · Potassium Electron Configuration (K) with Orbital Diagram
PH2 · Potassium Electron Configuration (K) with Orbital
PH3 · Potassium (K)
PH4 · How to Write the Electron Configuration for Potassium (K)
PH5 · Electron Configuration for Potassium (K, K+ ion)
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH8 · 3.3: Electronic Structure of Atoms (Electron Configurations)
PH9 · 3.1: Electron Configurations
PH10 · 2.6: Electron Configurations